Coelacanthi (lobe-finned fishes) >
Coelacanthiformes (Coelacanths) >
Latimeriidae (Gombessa)
Etymology: Latimeria: Taken from Miss Courtenay Latimer worker in the East London Musuem; she contributed to the update of the fish (Coelacanth, Latimeria chalumnae) (Ref. 45335); chalumnae: Chalumnae = the name of river Chalumna in South Africa, where the "first" Coelacanth was found (Ref. 45335).
Eponymy: Miss Marjorie Courtenay-Latimer (1907–2004) was the first Curator of the Museum at East London, South Africa, which was based on the Latimer family collection and holds the only remaining Dodo egg. [...] This is a toponym referring to the Chalumna River. It is ironic that such a famous marine fish is named after a river! The first known specimen was taken (1938) near the mouth of the Chalumna River, South Africa, by Captain Hendrik Goosen. (Ref. 128868), visit book page.
More on author: Smith.
Environment: milieu / climate zone / depth range / distribution range
Ecology
Marine; demersal; non-migratory; depth range 150 - 700 m (Ref. 38430), usually 180 - 250 m (Ref. 27564). Deep-water; 13°C - 25°C (Ref. 38429); 3°S - 34°S, 25°E - 51°E (Ref. 46167)
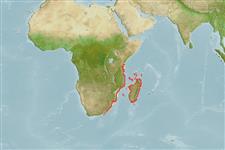
Indian Ocean: well known population off the islands of Grand Comoro and Anjouan in the Comoros. Other populations in South Africa (Ref. 11228, 38218), Madagascar (Ref. 26162), and Mozambique (Ref. 27415). Likely to occur as strays at islands like Astove and Cosmoledo (Ref. 1623).
Length at first maturity / Size / Weight / Age
Maturity: Lm 160.0, range 145 - 179 cm
Max length : 168 cm TL male/unsexed; (Ref. 30865); 200.0 cm TL (female); max. published weight: 95.0 kg (Ref. 26162); max. reported age: 48 years (Ref. 30865)
Dorsal spines (total): 8; Dorsal soft rays (total): 30 - 31; Anal spines: 0; Anal soft rays: 27 - 31. This species have the following characters: D VIII+30-31; A 27-31; P 29-32; V 29-33; C 20-25/35-38/21-22; LL 94-104; gills 4; gill rakers replaced by spiny tooth-plates. Head naked, the opercular bones exposed; gill cover expanded posteriorly and ventrally as a thick flap of skin; lower jaw with two large, overlapping gular plates; teeth conical, set on bony plates attached to palatines, ectopterygoids, and dentaries; maxilla absent (the structure at the side of the upper jaw that appears to be a maxillary bone is a thick fold of skin connecting the upper jaw to the rear of the lower jaw). Swim bladder elongate, filled with fat; intestine with spiral valve; osmoregulation involves retention of urea and trimethylamine oxide in the blood, but urea is not resorbed by the kidneys and excess salts are excreted by the rectal gland. In adults the brain is incredibly small, occupying only about 1% of the cranial cavity; but in the smallest juveniles, the brain completely fills the cranial cavity. Color in life: dark metallic blue, the head and body covered with irregular white or pale bluish spots. After death, the bluish color fades to dark brownish black.
Known as the living fossil. Inhabits steep rocky shores, sheltering in caves during the day (Ref. 38425), with as much as 14 individuals in a single cave (Ref. 38426). Foraging singly over open substrate at night (Ref. 38426), it drifts passively with the current or swims slowly with its paired fins and its second dorsal and anal fins (Ref. 38427). May travel as much as 8 km at night searching for food and retreats to the nearest cave before dawn (Ref. 38426). Preys on fishes and squid (Ref. 26162). Beryx, Polymixia, Symphysanodon, apogonids, a skate, an eel and a swell shark have been known to be eaten (Ref. 11228). Its main enemies are likely to be large sharks (Ref. 26162). Ovoviviparous, with as much as 5-29 young (Ref. 11228, 37171). Gestation period estimated at 3 years, which would be the longest known in vertebrates (Ref. 30865). A small relative gill area (Ref. 38428) restricts coelacanths to a life 'in the slow lane', drift-feeding at night in cold waters and resting in slightly warmer caves for food consumption during day time (Ref. 38429). Recently, Prof Hans Fricke and associates have succeeded in observing and filming Latimeria in their natural habitat. Using a two-man submersible, Fricke found several coelacanths in depths of 120-400 m on the barren lava slopes off Grand Comoro. Coelacanths have distinctive white markings, and this allowed recognition of individuals and tracking of their movements. During the day, Latimeria retreat to caves, with as many as 13 fish crowded together in a single cave. Several individuals occupy overlapping home ranges, and Fricke never saw any aggressive encounters between coelacanths. By resting in caves (were there are no strong currents) the coelacanths save energy and avoid encounters with large predators (deep-water sharks). After sunset, the coelacanths leave their caves and drift slowly across the substrate, presumably looking for food, within 1-3 meters of the bottom. On these nightly hunting forays, the coelacanth may travel as much as 8 km; and before dawn they shelter in the nearest cave. While searching for prey, or moving from one cave to another, Latimeria appears to move in slow motion, either drifting passively with the current and using its flexible pectoral and pelvic fins to adjust its position, or slowly swimming by a synchronous sculling movement of the second dorsal and anal fins. In slow forward swimming, the left pectoral and right pelvic fins move forward, while the right pectoral and left pelvic fins are pulled backward. This tandem movement of alternate paired fins resembles the movement of the forelimbs and hindlimbs of a tetrapod walking on land. Latimeria does not use its lobed fins for walking on the bottom, and even when they are resting in caves they usually do not touch the substrate. Like most slow moving fishes, the coelacanth can make a sudden lunge or fast start by means of a quick flip of its massive caudal fin. During its nightly foraging swims, Latimeria was often seen to perform head-stands, in which it rotates its body into a vertical position, with its head near the bottom and its caudal fin curved perpendicular to its body. It then held this position for two or three minutes at a time. This curious behavior may be used when it is scanning the bottom with its putative electoreceptive rostral organ, or it may be a reaction to the bright lights of Prof. Fricke's submersible (Ref. 38228).
Despite the lack of an obvious copulatory organ, the reproduction of Latimeria is of the type called "ovoviviparous", which means that it has internal fertilisation, and the fetuses are retained within the mother until they have grown large enough (36-38 cm) to fend for themselves. The eggs are enormous (9 cm in diameter and over 325 g in weight), and the huge yolk supplies all of the nutrients necessary for the growth of the embryo. In 1975, a large female coelacanth in the American Museum of Natural History was found to contain 5 young in individual compartments of the oviduct (uterus). They ranged in length from 301 to 327 mm and had well-developed teeth, fins and scales. Each fetus had a large, flaccid yolk sac attached to its chest. Dr Peter Forey of The Natural History Museum in London recently dissected one of these fetuses and found a 2 mm wide duct that leads directly from the yolk sac into the anterior part of the intestine. This yolk duct serves to move yolk from the yolk sac into the intestine where it is digested by the fetus. The same type of yolk transport into the gut via the yolk duct occurs in pups of ovoviviparous sharks. In some recent publications (Balon et al., 1988; Wourms et al., 1988; Bruton, 1989; Balon, 1991; Wourms et al., 1991) it was suggested (or even stated as a fact) that the reproduction of Latimeria involves "oophagy" or "embryonic cannibalism" (i.e., that the unborn pups feed on eggs or other siblings while in the uterus). According to Heemstra and Compagno (1989), there was no evidence to support this "oophagy" hypothesis, and Dr Forey's examination of a pup (from the original litter of 5 in the American Museum) found that its intestine was full of yolk (which is what one would expect with a direct connection between yolk sac and intestine) and contained "no trace of muscle fibres or anything else that might suggest that it had eaten a sib". Despite the misgivings of Heemstra and Compagno (1989), Wourms et al. (1991) again suggested that "Oophagy, the ingestion of supernumerary eggs by developing young, may well be the major source of supplemental nutrients for coelacanth pups." Speculating from a female that contained 19 ovulated eggs, they calculated that "19 embryos would occupy 7.0 meters of uterine space in a 2.0 meter fish" [the implication being that this is physically impossible]. They then concluded that "At the very most, such a fish could accommodate seven or eight developing embryos, and 11 or 12 eggs would then be superfluous-eggs ... [which] serve as nutrients for the embryos that survive to term". Then, in August 1991 a large pregnant female coelacanth, 179 cm long and weighing 98 kg, was caught by a trawler off Pebane on the northern coast of Mozambique (Bruton et al., 1992). This specimen was given to the natural history museum in Maputo, where it was dissected by the Director, Dr Augusto Cabral, who found that it contained 26 near-term pups, 31-36 cm in length. Thanks to the discovery and preservation of this Mozambique female, we now know that it is indeed possible for a coelacanth to have at least 26 pups in a litter, and the "superfluous-eggs" hypothesis of "oophagy" for the coelacanth is itself superfluous. Two pups from the Mozambique specimen were dissected by Heemstra and Greenwood (1992) and found to contain an internal yolk sac, which is the remnant of the large external yolk sac seen on the younger pups from the American Museum specimen. In the later stages of development, as the yolk supply dwindles, the external yolk sac apparently shrinks and is withdrawn into the body cavity. Some of the Mozambique pups had a small external yolk sac, and in others there was only a flat scar along the ventral midline to show where the yolk sac had been. In view of the large size (31-36 cm) and advanced development of the pups from the Mozambique female, the size at birth for Latimeria is probably about 35-38 cm (Ref. 38228). Juveniles are born after 13 months (Refs. 26162, 38222) or 3 years (Ref. 30865) of gestation period. Thus, females may give birth only every second or every third year.
Smith, M.M., 1986. Latimeriidae. p. 152-153. In M.M. Smith and P.C. Heemstra (eds.) Smiths' sea fishes. Springer-Verlag, Berlin. (Ref. 3185)
IUCN Red List Status (Ref. 130435: Version 2024-2)
Threat to humans
Harmless
Human uses
Fisheries: of no interest
Tools
Special reports
Download XML
Internet sources
Estimates based on models
Preferred temperature (Ref.
123201): 13.4 - 18.3, mean 15.6 °C (based on 16 cells).
Phylogenetic diversity index (Ref.
82804): PD
50 = 1.2539 [Uniqueness, from 0.5 = low to 2.0 = high].
Bayesian length-weight: a=0.01202 (0.00821 - 0.01760), b=3.03 (2.93 - 3.13), in cm total length, based on LWR estimates for this species (Ref.
93245).
Trophic level (Ref.
69278): 4.4 ±0.72 se; based on food items.
Generation time: 57.5 ( na - na) years. Estimated as median ln(3)/K based on 1
growth studies.
Resilience (Ref.
120179): Very Low, minimum population doubling time more than 14 years (K=0.02; tmax=85; tm=40-69; Fec=5).
Fishing Vulnerability (Ref.
59153): Very high vulnerability (89 of 100).